Write any non-zero formal charges on the appropriate atoms show all lone pairs of electrons as pairs of dots and all bond pairs as lines. Draw all reasonable resonance structures for each species.
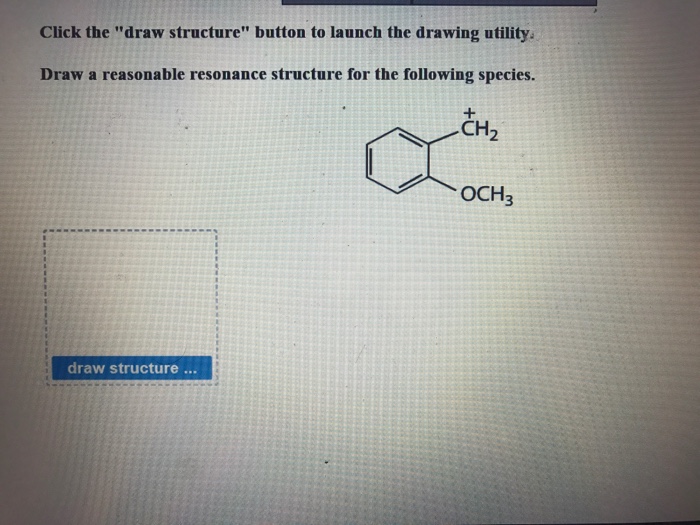
Solved Click The Draw Structure Button To Launch The Chegg Com
Convert each molecule into a skeletal structure.
. Draw all of the resonance structures for azide anion N3 and indicate the most stable o ne. A CH 3 2 CHCH 2 CH 2 CHCH 3 2 b CH 3 3 CCH 2 5 CH c CH 3 CHClCHOHCH 3 d CH 3 CH 2. Draw a reasonable resonance structure for the following species.
Draw resonance structures for the following species. Draw out all bonds to the oxygen atom in your answer. Draw a second resonance structure for each species.
Draw resonance structures for each radical. Draw all reasonable resonance structures for each species. Draw additional resonance structures for each species.
Below is an example of a resonance structure that would break the octet rule so it is a resonance structure that cannot be drawn. Draw all reasonable resonance structures for each species. A ClO 3 chlorate - Although the below seven are chemically reasonable Lewis structures.
Draw as many resonance structures as you can for the following species. Draw out all bonds to the oxygen atom in your answer. Draw all reasonable resonance structures for each species.
Then draw the resonance hybrid. Draw four additional resonance structures for the following cation. Therefore structure 2 is the most stable one.
Draw in all the carbon and hydrogen atoms in each molecule. Edit structure edit structure negative charge on carbon negative charge on oxygen View netails of I ast Check Answer ew Details Feb 24 2022 0201 PM. Draw all reasonable resonance structures for the following species.
When drawing resonance structures you must be sure to never exceed the octet rule for elements that follow this rule C N O F Cl Br I. 5 PAGES 234 - 235 Draw all chemically reasonable resonance structures for the following species. The answer between 1 and 2 choice D.
Draw as many resonance structures as you can for the following species. It is more preferable for negative formal charges to be on oxygen the more electronegative atom. For both structures 1 and 2 the formal charge is -1.
Draw additional resonance structures for each species.
Solved Draw A Reasonable Resonance Structure For The Following Species Draw Out All Bonds To The Oxygen Atom In Your Answer Ls Course Hero
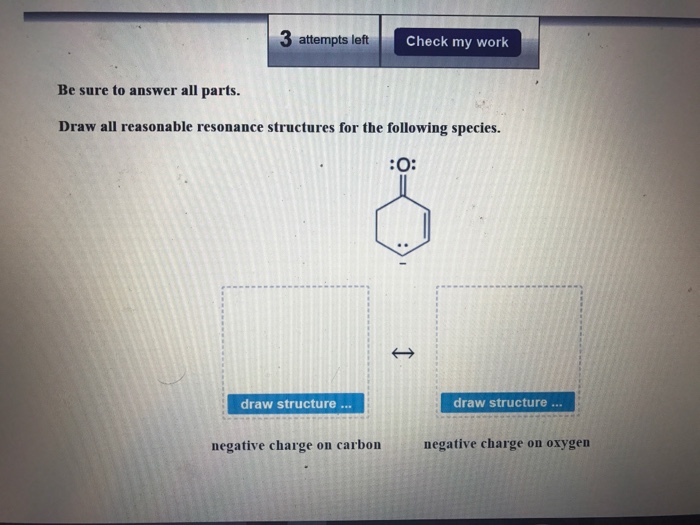
Solved Draw All Reasonable Resonance Structures For The Chegg Com

Solved Draw A Reasonable Resonance Structure For The Chegg Com
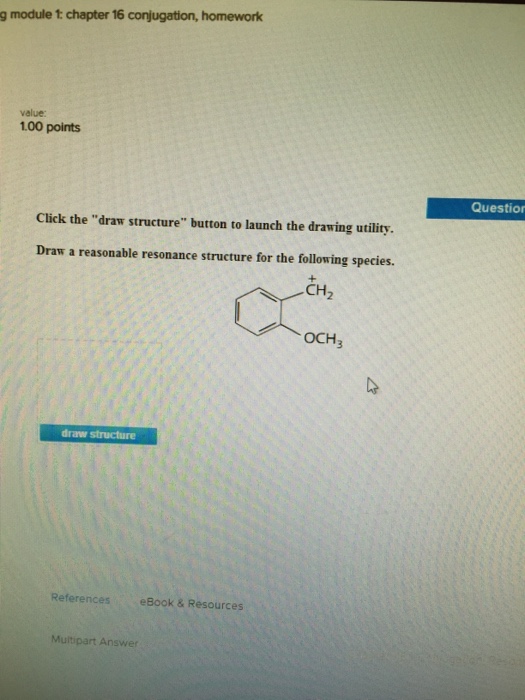
Solved Draw A Reasonable Resonance Structure For The Chegg Com

Draw A Reasonable Resonance Structure For The Following Species Study Com
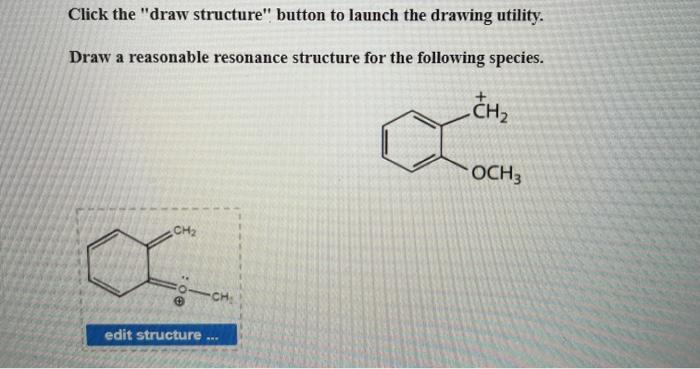
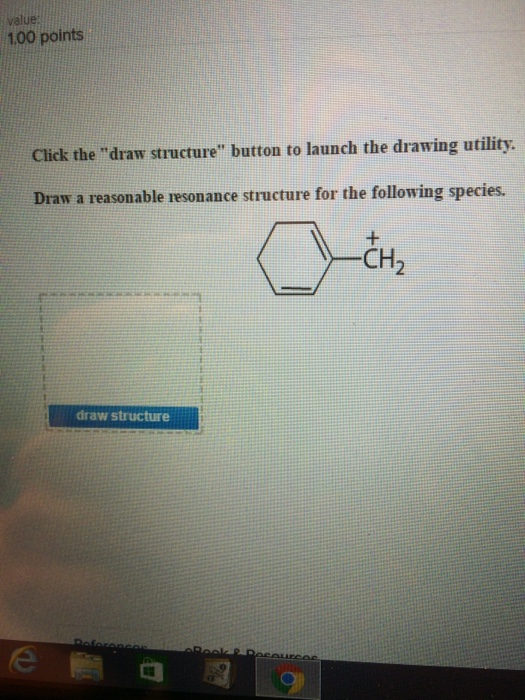
Solved Click The Draw Structure Button To Launch The Chegg Com
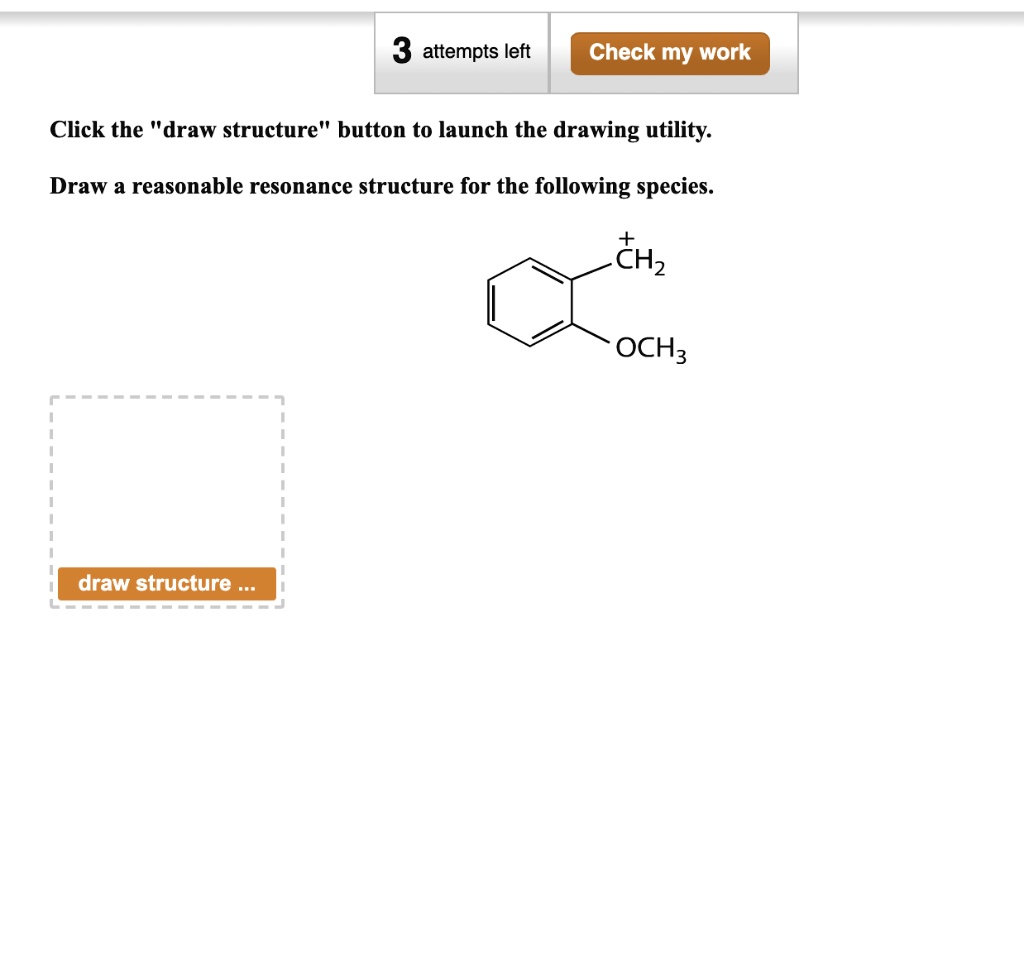
Solved 3 Attempts Left Check My Work Click The Draw Structure Button To Launch The Drawing Utility Draw A Reasonable Resonance Structure For The Following Species Ochz Draw Structure Thz
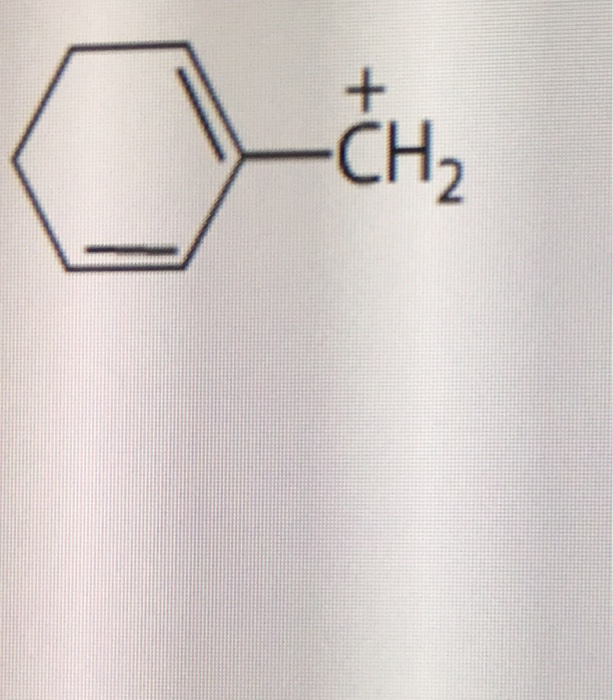
0 comments
Post a Comment